Adding microbial inoculants to improve temperature gain in a compost pile is examined.
Craig Coker
BioCycle February 2019
![]() Use of microbial inoculants (MI) to improve composting performance by accelerating the process, reducing odors, or saving money is a controversial issue, with professionals on both sides of the “Yes it works! No, it doesn’t work!” spectrum. Proponents claim microbial inoculation with specific bacteria and/or enzyme-producing microbes (enzymes are excreted chemicals that break down molecular compounds into simpler forms) can enhance the composting process by ensuring the right microbes are in the right temperature, oxygen and moisture regimes in the piles at the right times. Others claim it is a waste of money. This article focuses on MI intended to improve temperature gain in a compost pile rather than those MI that have been used to inoculate finished compost, such as Tirchoderma spp. bacteria, which have been shown to improve the fungal disease suppressive characteristics of compost-amended soils (Hoitink, 2006).
Use of microbial inoculants (MI) to improve composting performance by accelerating the process, reducing odors, or saving money is a controversial issue, with professionals on both sides of the “Yes it works! No, it doesn’t work!” spectrum. Proponents claim microbial inoculation with specific bacteria and/or enzyme-producing microbes (enzymes are excreted chemicals that break down molecular compounds into simpler forms) can enhance the composting process by ensuring the right microbes are in the right temperature, oxygen and moisture regimes in the piles at the right times. Others claim it is a waste of money. This article focuses on MI intended to improve temperature gain in a compost pile rather than those MI that have been used to inoculate finished compost, such as Tirchoderma spp. bacteria, which have been shown to improve the fungal disease suppressive characteristics of compost-amended soils (Hoitink, 2006).
Adding microbes to fresh incoming feedstocks to “accelerate” composting is not a new practice. In his seminal 1980 text on Compost Engineering, Roger T. Haug noted “Compost product can be recycled and used to condition the feed substrate.” Haug’s original premise was the benefit of recycled compost would be to offset the high moisture content of some substrates, like sewage sludges and food wastes, but operating practice has shown that recycling compost to the initial mixing phase also inoculates the pile with fresh, pile-acclimated microbes, so that thermophilic temperatures are realized more quickly. Some operators prefer not to recycle compost but do recycle overs from screening (which has compost bits adhered to the woody overs) and they see similar time-temperature response. Some call this more-rapid temperature rise, “accelerated composting”.
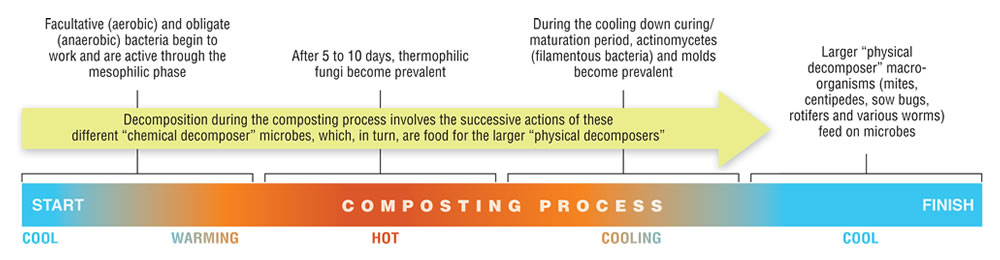 A remarkable aspect of composting is the more or less complete replacement of one set of microbes with another set, and then another, as the process works its way through the different temperature zones (Hubbe, 2010). A typical microbial succession has been described in the following terms: First, facultative (aerobic) and obligate (anaerobic) bacteria begin to work and are active through the mesophilic phase. Then, after 5 to 10 days, thermophilic fungi become prevalent. Following this period, during the cooling down curing/maturation period, actinomycetes (filamentous bacteria) and molds become prevalent. Decomposition within the compost involves the successive actions of these different “chemical decomposer” microbes, which, in turn, are food for the larger “physical decomposer” macro-organisms (mites, centipedes, sow bugs, rotifers and various worms).
A remarkable aspect of composting is the more or less complete replacement of one set of microbes with another set, and then another, as the process works its way through the different temperature zones (Hubbe, 2010). A typical microbial succession has been described in the following terms: First, facultative (aerobic) and obligate (anaerobic) bacteria begin to work and are active through the mesophilic phase. Then, after 5 to 10 days, thermophilic fungi become prevalent. Following this period, during the cooling down curing/maturation period, actinomycetes (filamentous bacteria) and molds become prevalent. Decomposition within the compost involves the successive actions of these different “chemical decomposer” microbes, which, in turn, are food for the larger “physical decomposer” macro-organisms (mites, centipedes, sow bugs, rotifers and various worms).
Evidence of this microbial action can be found in the variation of pH in a compost pile. The pH generally declines at the start of composting due to synthesis of organic acids by the bacteria (Golueke, 1972). For example, hydrolysis of acetate groups on hemicelluloses is an expected source of acetic acid (the smell of vinegar). However, as those acidic by-products are used by the microbial community, pH rises, elevating into the weakly alkaline range (pH = 7-8) due to the generation of ammonia.
Research into the field of composting has identified bacterial, fungal and other microbial strains that have pronounced degradation effects on certain types of feedstocks. We now know that the initial mesophilic temperatures are produced in the early stages of bacterially-dominated decomposition by species such as Pseudomonas sp., Streptococcus sp., Azobacter sp., Proteus sp., Bacillus sp., and subsequent thermophilic decomposition is enhanced by fungi such as Aspergillus, Emericella, and Penicillium, among others. Some research has examined deliberate inoculation of compost piles with specific species of bacteria.
Many composting feedstocks contain lignin, a complex organic polymer deposited in the cell walls of many plants, making them rigid and woody. Deliberate inoculation of piles with specific microbial species has been shown to improve the degradation of lignocellulosic materials (Vargas-Garcia, 2006), where cultures of Bacillus shackletonni, Streptomyces thermovulgaris, and Ureibacillus thermospaericus were introduced into cellulose- and lignin-rich aerated static compost piles. These species were selected on the basis of their capacity to excrete lignin-consuming enzymes. Lignin content in the final inoculated composts were 14.3 to 24.3 percent lower than in the uninoculated control piles. The study concluded that “the composting process can be improved by means of inoculation if the microorganisms used for this purpose are appropriate for the characteristics of the raw material.”
Effect On Organics In MSW
Van Fan, et.al. (2018) looked at 14 studies evaluating the effect of microbial inoculants (MI) on the organic fraction of solid wastes (OFMSW, mostly food wastes) and 11 studies of the effect on lignocellulosic wastes (LI, mostly yard wastes). This research team found that 38 percent of the MI studies reported a positive impact of inoculation on the composting of OFMSW and that 80 percent of the studies reported a positive impact on LI composting. They noted that the greater positive impact of inoculants on LI composting was due to the presence of enzymes breaking down the more decay-resistant lignin compounds. They also noted that those studies of MI addition to OFMSW composting reported more positive results when composting was carried out in controlled in-vessel conditions rather than in outdoors windrows.
Zhao, et.al. (2017) conducted a study to isolate degradative microbial strains from kitchen waste that includes discarded food residue and to develop a new microbial agent. One hundred and four naturally occurring strains were isolated from kitchen waste and 84 dominant strains were used to inoculate protein-, starch-, fat- and cellulose-containing media for evaluating their degradability. Twelve dominant strains of various species with high degradability potential (eight bacteria, one actinomycetes and three fungi) were selected to develop a compound microbial agent (labelled “YH”). Five strains of these species, including Brevibacterium epidermidis, Paenibacillus polymyxa, Aspergillus japonicus, Aspergillus versicolor and Penicillium digitatum, were new for kitchen waste degradation. YH was compared with three commercial microbial agents, Taiwan “Tiangeng” (TG), Hangzhou “Yilezai” (YLZ) and the Japanese effective microorganism inoculant (EM), by assessing their effects on reduction, maturity and deodorization.
Reduction of kitchen waste mass is an important indicator of MI efficacy. The results showed that YH achieved the greatest mass loss, which decreased about 65.87 percent after 14 days. The other MI strains reduced mass 42 percent (CK), 61.5 percent (TG) 59.7 percent (YLZ) and 60.8 percent (EM). The YH agent inhibited ammonia (NH3) and hydrogen sulfide (H2S) emissions significantly during the composting process. The concentration of NH3 decreased from 7.1 to 3.2 ppm; H2S decreased from 0.7 to 0.2 ppm.
Not all studies have reported positive effects of inoculation. One theory about this is that inoculants are introduced into mixes that already contain diverse communities of organisms, each of which can multiply rapidly when favorable conditions are established (Golueke, 1972). Karchanawong (2013) looked at the effects of microbial inoculation with two commercial inoculants popular in the Thai market (trademarked EM and LDDI, a Thai blend of fungi, actinomycetes and lipase-secreting bacteria) and mature compost on the composting of household organic wastes (food waste and leaves). Feedstocks were composted for 94 days. The mean temperatures observed in all piles during the composting period ranged from 39.5°C to 40.0°C (103-104°F) and 28.6° to 28.7°C (83.5°-83.7°F), but were not significantly different from each other. Researchers found that C:N ratios of compost in the unseeded pile stabilized after 81 days and in the inoculated piles in 67 to 74 days.
Inoculated Modified Static Aerated Pile
One commercial microbial inoculant product on the U.S. market is Harvest Quest (HQ). Mussari (2013) treated dewatered sewage sludge mixed with green waste (1:3 ratio, v/v) with the HQ inoculant in an aerated static pile composting facility, using a 60-foot long pile aerated with a 5 hp blower. The material was allowed to continue through the “active” phase of composting for 25 days and then was turned using a front-end loader. The pile was reformed and allowed to reheat for 24 hours. The blower was then turned on at a low speed and the pile was “force-cured” for 7 days.
At the end of 32 days, the material was screened using a three-eighth-inch trommel screen and the finished compost was placed in a pile for storage. The “overs” accounted for approximately 60 percent of the final mass and were reused as bulking material in subsequent piles. Process to Further Reduce Pathogens goals (PFRP, > 131°F) were met within 5 days of pile formation. Vector attraction reduction (VAR) requirements were met within 16 days of pile formation. The study did not compare treated pile results to untreated piles.
The Harvest Quest product is a proprietary blend of enzyme-producing bacteria and fungi that are claimed to self-inoculate a compost pile to produce compost in less time and with less odor (due to fewer turns of a turned windrow composting approach or less need for fan-forced/induced aeration in an aerated static pile system). The HQ-inoculated method is marketed as the Modified Static Aerated Pile (MSAP) composting approach.
An early proponent of microbial inoculation is A1 Organics, which has been involved with, and has used, HQ since the early testing days back in 2000. A1 Organics is a multisite regional composter in Colorado, handling around 50,000 wet tons/year each of biosolids and food wastes. Bob Yost, Chief Technical Officer for A1 Organics, explains that A1 introduces the HQ product on the surface at one to three places along a windrow (depending on length), then covers the windrow with a 6- to 8-inch layer of freshly composted unscreened MSAP output. Operators have a basic formula (based on the tonnage of biosolids in the windrow) from which they derive the bulk volume of HQ to apply to an individual windrow.
The windrow is not touched for 30 to 45 days but temperatures are monitored consistently at 10-, 20-, 30- and 40-inch depths at numerous points along the windrow. “We see PFRP temperatures (>131oF) within 24 to 48 hours in the outer edges of the windrow, with temperatures rising in the core shortly thereafter,” notes Yost. “The average temperature at each depth must exceed 131°F for a minimum of 72 hours to meet PRFP requirements. We always see those elevated temperatures for extended periods of time well beyond the minimum. We have never failed a pathogen content test in finished compost.”
He adds that per Colorado’s regulations, in addition to the finished compost composite test, A1 is required to take 7 individual samples per month from the finished screened product for individual fecal tests. “We have taken an estimated 1,450 such samples since we started with MSAP,” he says. “There have been a few times that an individual sample may have exceeded the 1,000 mpn (most probably number) target, but the required geometric mean average has passed.”
Harvest Quest has a production facility at one of A1 Organics’ composting sites where it creates a media from material it brings to the site. HQ inoculates that media to profligate its target bacterial contained in the media. The final product is shipped to other clients from that location in bulk bags.
PFRP Equivalency
One potential issue associated with use of a microbial inoculant product that allows for less windrow turning is the adoption of the Federal Process to Further Reduce Pathogens (PFRP) and vector attraction reduction (VAR) standards for the beneficial reuse of sewage sludges (40 CFR Part 503). The reduced turning frequency of MSAP for windrow composting puts the method at odds with the PFRP definition in the federal, and some state, regulations. The Florida Department of Environmental Protection (FDEP), for example, allows use of the MSAP method as a modification of the PFRP windrow process, not a separate equivalency for pathogen reduction (Schinella, 2016).
In 2003, U.S. EPA Region 9 issued an opinion to Nursery Products in San Clemente, California, that use of the HQ product “successfully met the regulatory requirements of 40 CFR Part 503 and is considered a PFRP [Class A] composting method when used at your facility. The process is not a windrow process as defined in 40 CFR 503 Appendix B and differs from a standard Static Aerated Pile process in that air is not mechanically forced through the piles.”
In 2009, the Colorado Department of Public Health and the Environment wrote to EPA Region 9 about the use of MSAP at A1 Organics: “Following a year of extensive testing by A1 Organics, the MSAP method was approved by the Hazardous Materials and Waste Management Division of the Colorado Department of Public Health and Environment (“the Department”) as an acceptable composting method. The Department’s Water Quality Control Division has approved the MSAP method, Colorado Regulations No. 64.12.B(7)(b)(i), for both static aerobic piles and windrowing for composting of biosolids.”
Also, in 2009, Dr. James Smith at EPA’s Office of Research and Development wrote to CompostUSA in Silver Springs Florida about use of MSAP in seeking Pathogen Equivalency Committee approval: “In my opinion, you should work with USEPA Region 4 and the State of Florida to determine the conditions that you must operate under to have a Class A, Alternative 5 (Process to Significantly Reduce Pathogens (PSRP)) Process. If you are unable to meet their requirements with your process and still feel that your process is PFRP equivalent, then I suggest you follow the (Pathogen Equivalency Committee) procedures.”
While these regulatory citations refer to the use of MSAP in composting biosolids, the same regulatory testing and approval approach may be needed in those states where the specific time-temperature requirements of PFRP and/or VAR have been embodied in state solid waste composting regulations.
Summary
Inoculating fresh feedstocks with acclimated microbes from the composting process by either mixing in overs and/or recycling some compost is a tried and true method of bringing compost piles up to acceptable temperatures quickly. Adding specific mixes of microbes to accelerate the temperature rise to thermophilic levels (and thus, “accelerate” composting) has been demonstrated, both in the U.S. and overseas.
In the end, like everything else about organics recycling, it boils down to economics. For a yard trimmings composting operation looking to make product faster, the costs of inoculants might make sense due to cost savings from reduced windrow turning. For an aerated static pile food waste composting operation in a state with rigid regulatory requirements about PFRP/VAR, it may not, depending on the conditions imposed by regulators to keep proving it is an equivalent method to PFRP/VAR.
Craig Coker is a Senior Editor at BioCycle and CEO of Coker Composting & Consulting near Roanoke, VA. He can be reached at ccoker@jgpress.com.