Odor emissions from composting sites has prompted scientists in England to rethink the way conditions are monitored using principles developed by Victorian chemists 200 years ago.
Nick Sauer and Eric Crouch
BioCycle December 2013
 Composting is, by definition, a predominantly aerobic process. Failure to provide sufficient oxygen for aerobic degradation by microbes results in slow maturation rates and excessive odors. For industrial composting activities, supplying oxygen costs money. This may be through extra land use for low passively ventilated windrows, or power and equipment for forced ventilation systems. Therefore, carefully monitoring oxygen levels to optimize the composting process while minimizing odor pollution is necessary.
Composting is, by definition, a predominantly aerobic process. Failure to provide sufficient oxygen for aerobic degradation by microbes results in slow maturation rates and excessive odors. For industrial composting activities, supplying oxygen costs money. This may be through extra land use for low passively ventilated windrows, or power and equipment for forced ventilation systems. Therefore, carefully monitoring oxygen levels to optimize the composting process while minimizing odor pollution is necessary.
Progressive operators may already be using commercial or home-built probes to check the supply of oxygen in composting windrows or tunnels. That is an important step forward, but to get full value from these measurements, the influence of temperature needs to be considered. First, there is an inbuilt error in the oxygen measurements that increases at higher temperatures. This can be corrected using Dalton’s Law, courtesy of English Victorian chemist, John Dalton.
Second, microbes live in the water layer coating composting particles, not in air. The limited amount of oxygen that can dissolve in water is further reduced at high temperatures. Soluble oxygen levels can be calculated using Henry’s Law, developed by another Victorian chemist, William Henry.
This article explains each of these calculations and provides simple methods for assessing the availability of oxygen. A compost operator is then in a much better position to judge the health and productivity of the site’s composting system.
Volumetric Corrections
Dalton’s law shows that at very high composting temperatures — 176°F (80°C) — saturated air contains nearly 50 percent water vapor, while the saturation moisture level drops to less than 10 percent at a more moderate 113°F (45°C). Since air flows through a composting material quite slowly and about half of the weight of composting material will be water, any air contained in the compost pore spaces will be saturated.
This means that the highest oxygen level which can be achieved at 176°F (80°C) will be about 11 percent, compared with 21 percent in cool ambient air. Of course, in actively composting material, oxygen will be continuously used up by microbial activity and levels may be much lower.
Measuring oxygen in this hot wet environment poses a problem. The inserted tip of the compost probe will quickly assume the temperature of the compost windrow. However, the exposed end (and the oxygen detector) will remain at ambient temperature. As air is removed from a compost windrow, it will be cooled by the cold probe between the pile and the instrument. As it cools, moisture will condense in the sample tube as liquid water. If the detector is at 59°F (15°C), for example, then air reaching the detector will contain only 1.6 percent moisture. With no oxygen consumption at all, the detector will incorrectly read around 21 percent oxygen because most of the moisture will have been removed, causing the oxygen to be more concentrated.
Fortunately, it is a relatively simple matter to correct oxygen readings taken from samples of extracted air that have been cooled. To make the results of these calculations more readily available, Table 1 provides oxygen corrections for hot saturated air. Use the measured compost temperature and oxygen meter readings to look up the corrected oxygen concentrations.
Oxygen Solubility
So far so good, but composting microbes don’t live in the air spaces within the windrow; they live in the water layer, or biofilm. Furthermore, oxygen does not dissolve in water very well. Even a cool, well aerated trout stream will contain less than 10 micrograms of oxygen per liter of water (µg/l). Micrograms per litre is also referred to as parts per million (ppm) because there is a ratio of one gram of oxygen to every million grams of water. For comparison, 10 ppm is the same as 0.001 percent by weight.
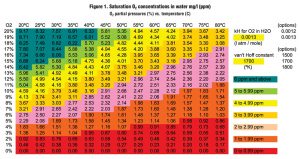
Henry’s law shows that as temperature goes up, the amount of oxygen that can dissolve into water goes down even further. For a bowl of water exposed to ambient air at 113°F (45°C), the concentration of dissolved oxygen will have dropped to about 6 ppm. The same bowl of water at 176°F (80°C) will contain only about 3.5 ppm oxygen. These ppm concentrations can be looked up in Figure 1.
A concentration of 3.5 ppm is healthy for aerobic composting microbes, but that is for water with direct access to ambient air at 21 percent oxygen. Compost air won’t have that much oxygen because it is both consumed by microbes and displaced by water vapor. After applying the volumetric correction, it is common to find very low oxygen levels in compost air.
An example will provide a clearer illustration of this basic principle of composting. If a meter reading indicates 5 percent oxygen at a moderate compost temperature of 113°F (45°C), the true oxygen level, utilizing Dalton’s Law, can be looked up in Table 1. Find 5 percent across the top row (O2% as read) and 45 percent down the first row (Compost Temp °C). Where they meet shows the corrected (actual) oxygen level of 4.6 percent.

Carefully monitoring oxygen — provided through forced ventilation, passively aerated windrows or pile turning — helps optimize the composting process while minimizing odor pollution.
The next step is to consider how much oxygen can dissolve in water at this temperature. In Figure 1, 45ºC is visible in the top row, but there is no precise value for 4.6 percent down the first column. Even so, an approximate value between 1.16 ppm for 4 percent oxygen and 1.45 ppm for 5 percent oxygen can be extrapolated. An Excel add-in and an online calculator have been developed to do these calculations (http://www.compostmanager. com/en/o2-calculators). Use either Centigrade or Fahrenheit, whichever is most convenient.
The online calculator shows our example 5 percent oxygen reading at 113ºF (45ºC) to have a corrected oxygen reading of 4.6 percent (the same as Table 1) and an oxygen saturation level of 1.34 ppm. This is between the Figure 1 lookup values of 1.16 and 1.45 as expected. If the compost temperature is higher, e.g., 176ºF (80ºC), the same 5 percent oxygen reading would show a corrected oxygen concentration of 2.7 percent and a saturation level of only 0.47ppm.
While all this measurement and calculation helps to understand what is going on and indicates the highest possible oxygen levels that might be found in the biofilm, there is more to the story. By definition, microbes live in a very small environment. They may live in a neighborhood near the surface of a biofilm, where there is easier access to oxygen. Or they may live deep in the biofilm close to the food source, but far away from their oxygen supply.
Calculating the surface oxygen concentrations in compost biofilms takes us a step closer to understanding the environment in which microbes live. Unfortunately, these calculated values don’t directly indicate whether the environment below the surface is predominantly aerobic, whether there is enough oxygen to reduce odors or whether the maturation process is actually being optimized. It may be that at high temperatures, oxygen demand is very low because microbial activity is limited and composting is happening very slowly. If that is the case then core oxygen levels may be similar to saturation levels at the particle surface. However, these calculations do provide a more useful yardstick to compare conditions at sites with odor problems to sites where odor is more under control.

The inserted tip of the compost probe quickly assumes the temperature of the compost windrow while the exposed end with the oxygen detector remains at ambient temperature. It is relatively simple to correct the oxygen readings.
Dissolved Oxygen Levels
Data collected using the CompostManager system from several sites in the United Kingdom have been analyzed with a view to determining the minimum required levels of dissolved oxygen in order to avoid excessive odor. By selecting data from well-performing sites without significant odor problems, it appears that if dissolved oxygen levels in windrows are above 1 ppm most of the time, there are unlikely to be major odor problems. By contrast, data from failing sites with significant odor problems consistently showed dissolved oxygen levels below 0.5 ppm.
As these results do show a significant difference between odorous and nonodorous conditions and the dataset is large (over 100,000 individual readings), this would certainly suggest that dissolved oxygen levels are an invaluable tool for monitoring and reducing odorous conditions in compost. Indeed, trends in this value have successfully been used to identify potentially problematic batches on sites that are otherwise performing well.
However, it is difficult to argue that this inductive approach alone provides the basis for a conclusive threshold limit for dissolved oxygen. Additional research is needed into the link between dissolved oxygen levels and scientifically measured odor emissions from composting materials and systems.
Conclusions
With the possible exception of short-term heat treatments designed to kill pathogens and weed seeds, it is good practice to manage composting activities with enough oxygen to maintain aerobic conditions. As is commonly accepted, failure to maintain sufficient levels of oxygen will result in odor pollution and poor composting performance.
Oxygen monitoring can be used to assess whether the composting process is under control, but direct readings alone are likely to be misleading. Using the online calculator (or the tables) to determine saturated oxygen conditions in the water biofilm will provide a much better indication of living conditions for microbes within actively composting materials.
We have a general idea of how much dissolved oxygen is necessary to minimize odor pollution. However, further work is necessary to establish this more confidently and in a manner that takes into account variables such as compost moisture percentages and readily available carbon. Also, it seems likely that higher oxygen levels may be required to optimize composting performance. Saturated dissolved oxygen (in ppm) is a much more powerful indicator of favorable composting conditions than raw percent oxygen measurements.
Nick Sauer is a technical advisor with the Environment Agency for England (www.environment-agency.gov.uk). He leads a team of technical staff who advise on the monitoring and control of odor at waste sites (nick.sauer@environment-agency.gov.uk). Eric Crouch is a research and development scientist for CompostManager, a UK-based company that supplies unique process control software and monitoring equipment for composting systems (eric.crouch@compostmanager.com).