New series looks at odor management during composting and anaerobic digestion. First article explores how odors are created, odor sources and regulatory considerations. Part I
Craig Coker
BioCycle April 2012, Vol. 53, No. 4, p. 25
Over the next several months, BioCycle is publishing a series on odor management at composting and anaerobic digestion facilities. Failure to control and manage odors is the single biggest cause of adverse publicity, regulatory pressures and facility closures in the organics recycling industry. The article series examines the intricacies of odor management — how and where odors are generated, how they are measured and perceived, management through good process control as well as with technology, and addressing the neighbor and community relations of organics recycling odors. This first article explores how odors are created, odor sources and approaches to regulating their emissions and control.
Aerobic composting and anaerobic digestion facilities have one thing in common: they manage the process of decomposition. Decomposition begins immediately after the death of a living plant or animal, whether that’s an orange plucked from a fruit tree, an animal rendered to feed people, or a shrub branch pruned by an avid gardener. Decomposition is a biological and chemical process whereby complex biochemical compounds are broken down into their constituent building blocks. For example, acetic acid (vinegar) decomposes aerobically into water (H2O), carbon dioxide (CO2) and ethane (C2H6)).
Aerobic decomposition is the cornerstone of composting. Aerobic composting is an oxidation process, whereby decomposition raises the oxidation state of the building blocks. Oxidation — the same process that turns an apple skin brown, a bicycle fender rusty or a copper penny green — is defined as the interaction between oxygen molecules and all the different substances they may contact, from metal to living tissue. Oxidation occurs on a molecular level, but we see it when the free radicals formed by oxidation break away (rust flakes, copper oxide particles, brown spots on fruit). In the vinegar example, the three decomposition products are said to have a higher oxidation state than the acetic acid.
In food scraps composting, the main components are proteins, carbohydrates and fats, which contain various combinations of carbon, hydrogen, oxygen, nitrogen and sulfur. Decomposition of these compounds follows a well-evolved sequence of events (Figure 1).
An odor is a volatile chemical gas. Volatility is the tendency of a substance to vaporize, which is proportional to a substance’s vapor pressure. At a given temperature, a substance with higher vapor pressure vaporizes more readily than a substance with lower vapor pressure. As an organic material decomposes, the mix of volatile compounds change, so the mix of vapor pressures changes, which in turn can change the characteristic odor. Some odors are produced by the biological changes in compounds by microorganisms; others are due to chemical changes in the composting pile (e.g., raising the pH of a pile by adding wood ash will shift the equilibrium between gaseous ammonia and soluble ammonium in favor of the gaseous ammonia, thus causing an ammonia odor). The major odor-causing compounds in composting are sulfur-, nitrogen-, and carbon-based. Table 1 lists odorous compounds and their distinguishing odor characteristic(s).
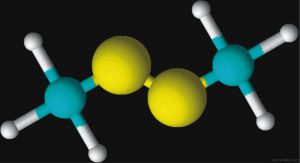
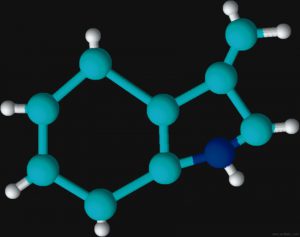
Figures 2 and 3 are schematic representations of two odor molecules, dimethyl disulfide (DMDS) and skatole. The odor detection threshold (DT) is the lowest concentration of a certain odor compound that is perceivable by the human sense of smell. DMDS has a detection threshold of 2.2 x 10-3 parts per million (ppm) while skatole’s DT is over 1,000 times less (5.6 x 10-6 ppm). As a basis for comparison, a concentration of one ppm would be like one inch in 16 miles.
Factors Influencing Odor Generation
A composting pile is a highly dynamic ecosystem, constantly changing over the 21- to 60-day life of the active composting phase. Factors that influence odor generation include: feedstock composition, metabolic activity rates of the decomposers doing the work, availability of the nutrients in the feedstocks to the microbes, how well mixed the feedstocks are, and several physical factors, such as moisture content, particle size, oxygen content and diffusion, and temperature.
Different composting feedstocks have different odor characteristics, or profiles. Biosolids have around 0.8 percent sulfur content (8,000 ppm) and anaerobic conditions in the wastewater conveyance systems allow the reduction of the organic sulfur contained in amino acids in human waste (cysteine, methionine) to foul-smelling compounds like dimethyl disulfide, methyl mercaptan (the odorant they put in natural gas) and hydrogen sulfide. Feedstocks found in typical yard trimmings composting operations contain terpenes — the primary constituents of the oils found in plants and flowers, including limonene, menthol and alpha-pinene. While some find odors from yard trimmings composting unacceptable, most fully aerobic green waste composting odors are the sweetish earthy smells most composters find to be a pleasant smell.
Degradability of a feedstock also influences odor generation. For example, seafood processing residuals are highly degradable and consume a lot of oxygen very quickly as they decay. (Highly degradable residuals reach a complete oxidation state faster than more slowly-degradable materials; for example, a rotting piece of fruit versus a rotting piece of wood.) Two problems occur with highly degradable materials. The first is that oxygen is consumed faster than it is replenished, with the formation of anoxic conditions. Second, the initial odorous by-products of decomposition (methylamine) don’t oxidize to less odorous forms (ammonia) fast enough, resulting in a build-up of the first-stage odorous by-products.

The quality of feedstock mixing also influences odor generation. With food scraps composting, the round nature of many fruits and vegetables makes it hard to keep them in the pile, much less adequately mixed.
The quality of feedstock mixing can also influence odor generation. In an ideally mixed pile, a thin layer of the nitrogenous feedstock would evenly cover the carbonaceous amendment in the pile. Typically, however, it is impossible to mix feedstocks that finely or completely. More often, wetter heavier materials (like sludges and manures) will form “balls” that simply roll around in the mixing device. These clumps of largely nitrogenous material will not compost completely and if they are broken open by mechanical agitation during composting, they will release odors. With food scraps composting, the round nature of many fruits and vegetables makes it hard to even keep them in the pile, much less adequately mixed.
Sources Of Odors In Composting
Decomposition began when the living material now making up the feedstock died. So odor formation begins almost immediately with the onset of decomposition. Virtually every composting facility has had to deal with a very odorous feedstock coming into the receiving area.
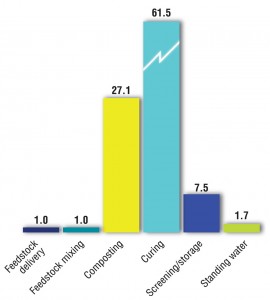
A previous study (Epstein and Wu, 2000) correlated odor emissions by source at a windrow composting facility. Figure 4 illustrates their findings.
“It should be noted however that odor dispersion from a site is not simply a matter of the number of odor units generated; there are many parameters to consider including source dimensions, topography, and the hours of emissions. Odor balances also do not take odor intensity into account; for example, while new compost piles may not produce the highest number of odor units, the intensity of the odor generated may be higher because of the types of compounds formed during the early stages of composting. Higher intensity odors are detectable at lower concentration and therefore have a relatively higher potential to cause odor impacts.”
Perceptions And Regulation Of Odors
Odor science is a very precise field of endeavor. Measurements are made in units of micrograms per cubic meter (1 µg/m3 =1.6 x 10-9 lbs/CY). Impacts are measured in “dilutions-to-threshold” (the number of volumes of clean air needed to dilute an odor to its recognition threshold). And unusual terms like “olfactometry” are used (the science of measuring the acuity of the sense of smell). Yet, those affected by odor episodes at composting facilities inevitably respond in purely emotional contexts, with complaints using words like “disgusting,” “nauseating” and other highly-charged descriptors.
It is human nature that our reactions to odors are subjective and emotional. After all, an odorant is an air pollutant, and few things frighten people more than breathing in air that is “contaminated” with a chemical, particularly if their reaction to that chemical is unpleasant. What makes regulation and management of odors so difficult is that no two people will react the same way to an odorant. Everyone is different in how they perceive an odor, how pleasant or unpleasant they think it is, and how strong the odor is before it becomes noticeable, annoying or truly objectionable.
As a result, the majority of odor laws in the U.S. are written around a nuisance standard. Under the common law, persons in possession of real property (land owners, lease holders, etc.) are entitled to the quiet enjoyment of their lands. If a neighbor interferes with that quiet enjoyment, either by creating smells, sounds, pollution or any other hazard that extends past the boundaries of the property, the affected party may make a nuisance claim. For a “bad” odor to be considered a nuisance, it must alter one’s daily activities. Simply smelling an odor is not a nuisance; therefore, in most cases single, mild, short-lived odor events are not considered nuisances. However, if the same event is not short-lived, even a mild odor could be considered a nuisance.
State environmental regulators have a difficult time enforcing odor regulations against any source, not only composting facilities. To start with, they must detect the odor at the point of complaint, and with sufficient strength and repetition to give credence to the complaint. Even then, the regulator may not perceive the odor with the same distaste as the complainant.
Many regulators will try to get complainants to be systematic in recording their observations of odor episodes. One program was developed by the Texas Commission on Environmental Quality (TCEQ), and is known as FIDO.
FIDO stands for Frequency, Intensity, Duration and Offensiveness. The steps in the FIDO process include:
1. Characterize the odor to determine which Offensiveness table to use (Not Unpleasant to Highly Offensive).
2. Assess the Intensity of odor (Very Light to Very Strong).
3. Determine the total Duration of the odor(s) (1 minute to 24 hours).
4. Evaluate the Frequency of odor occurrence (Single Occurrence to Daily).
5. Identify the block on the chart that corresponds to the information from Steps 1-4 and determine if a nuisance condition exists.
TCEQ developed four different “Offensiveness” charts to help regulators decide if a nuisance had indeed been created. Table 2 illustrates a chart where odors are characterized as highly offensive.
Odor management and control in organics recycling may be the single most important responsibility of facility managers. It is a highly complex topic. Part II of this series, to appear next month, focuses on how process control can manage odors.
Craig Coker is a Contributing Editor to BioCycle and a Principal in the firm Coker Composting & Consulting (www.cokercompost.com),near Roanoke VA. He can be reached at cscoker@verizon.net.
References
Epstein, E. and N. Wu, “Planning, Design, and Operational Factors that Affect Odor Control at Composting Facilities”, Composting in the Southeast, Charlottesville, VA, 2000.