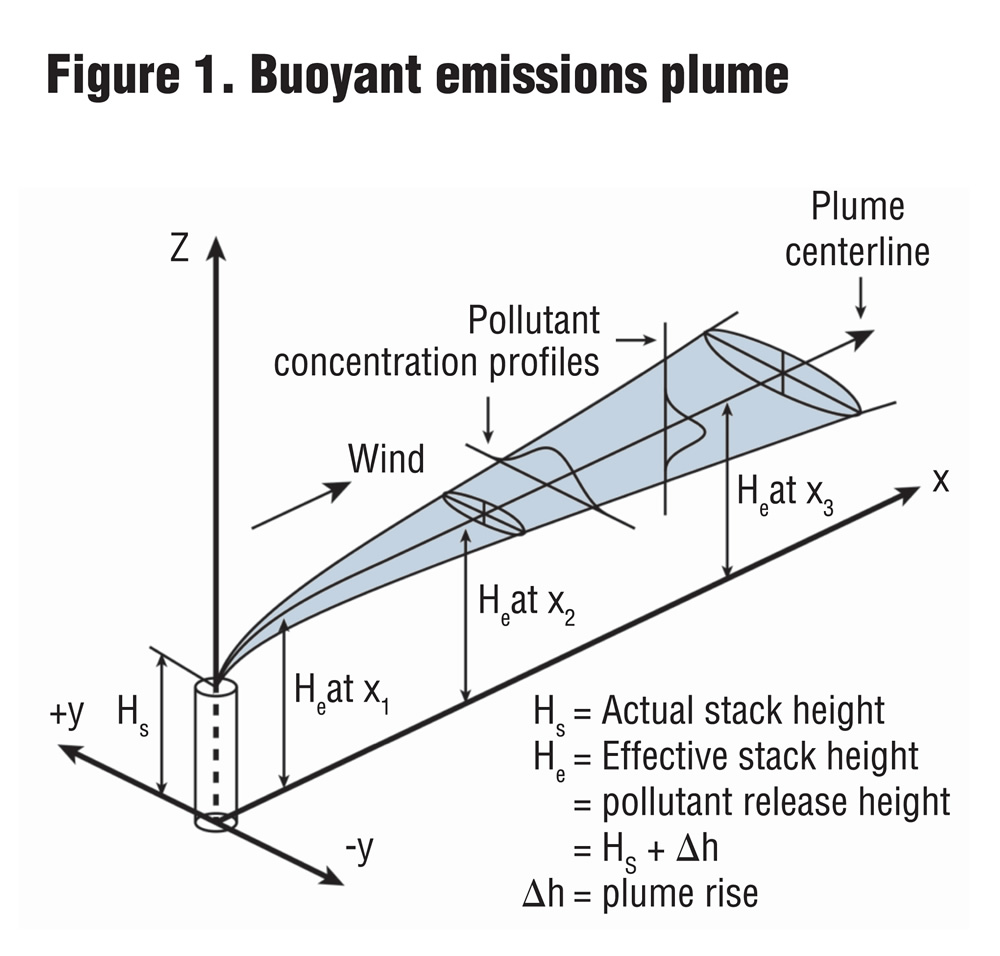
Dispersion is the phenomenon where odor concentrations are reduced to below recognition or detection thresholds. It is influenced by meteorological conditions and topographic effects. Part VI
Craig Coker
BioCycle June 2013, Vol. 54, No. 6, p. 26
Craig Coker resumes his article series on odor management with a focus on dispersion. Previous articles, available in the BioCycle archives (www.biocycle.net), covered how and where odors are generated, measured and perceived; how they are managed through good process control as well as technology; and public outreach related to organics recycling odors.
No matter how well planned, sited or operated, composting facilities are always at risk for an off-site odor episode that can create challenges such as negative publicity, regulatory intervention and lawsuits. This article series, which began in 2012, has been dedicated to helping composters understand the intricacies of odor management. Part VI looks at the fundamentals of how odors behave in the environment.
As discussed in Part I (BioCycle, April 2012, p. 25), an odor is an air pollutant. It is a chemical emitted in the gaseous vapor phase and behaves in the atmosphere like other gasses. Air pollutant emissions can be characterized by type, source and elevation. Types of emissions include point sources (e.g., a smokestack), line sources (e.g., cars on a highway), area sources (e.g., from a forest fire), or volume sources (e.g., from a paint shop with multiple roof vents). Emissions from composting facilities are usually considered area or volume sources. Emissions can be further characterized as mobile versus stationary and urban versus rural, as the urban heat island effect makes the atmosphere above cities more turbulent, which affects dispersion. The elevation of an emission is another discriminant, with categories of surface, or ground level, near surface and elevated surface.
Gaseous or particulate emissions from a continuous source are emitted as a “plume” of material, so-called because the flow of emissions resembles a feather in shape and appearance. The emissions of smoke or steam from an elevated source are a visible example of a plume. Emissions from an intermittent source are considered “puffs” where a cloud of material is released and moves downwind.
Dispersion is the phenomenon where odor concentrations are reduced to below recognition or detection thresholds. Odor dispersion could be viewed as “dilution is the solution to pollution.” Plume or puff dispersion is affected by meteorological conditions, terrain, building downwash and deposition (e.g., contact with ground, vegetation or rain). Meteorological parameters that influence pollutant dispersion are wind speed and direction, as well as vertical thermal stratification or mixing. The pollutant concentration is inversely proportional to the wind speed, so that if wind speed doubles, pollutant concentration is cut in half. This is due mainly to the accelerated transport of the plume’s constituents by the wind. Moreover, turbulent mixing increases with growing wind speed, which enhances dispersion.
Atmospheric Dispersion
Atmospheric stability plays the most important role in the transport and dispersion of odors. It can be defined as the atmospheric tendency to reduce or intensify vertical motion or alternatively, to suppress or augment existing turbulence. The amount of turbulence in the atmosphere has an effect on odor dispersion by mixing uncontaminated air with the odor-laden air, reducing its concentration. Turbulence is caused by vertical motions of air in the atmosphere (convective turbulence) and by horizontal motions of air due to winds (mechanical turbulence). Vertical motions can be attributed to high and low pressure systems, air lifting over terrain or fronts and convection. Convective turbulence is caused by these rising and falling parcels of air (consider this analogous to puffs of odorous air emitted from a composting pile). Normally, the air closest to the earth’s surface is warmer than the air aloft, due to solar heating of the earth. This warmer parcel of air rises. The extent to which an air parcel rises or falls depends on the relationship of its temperature to that of the surrounding air. As long as the parcel’s temperature is greater, it will rise; as long as the parcel’s temperature is cooler, it will descend. When the temperatures of the parcel and the surrounding air are the same, the parcel will neither rise nor descend unless influenced by wind flow.
The rate at which the temperature of the air changes with elevation is called the lapse rate and is normally 3o-4o F/1,000 ft. of elevation, but it varies widely with location and time of day. If the temperature decreases with elevation, it is called a negative lapse rate; if it increases with elevation, it is called a positive lapse rate. The lapse rate is important to vertical motion in the atmosphere since surrounding air temperature determines whether a parcel of air rises or falls. Atmospheric lapse rates are normally negative. When this normal cycle is present, it is considered an unstable air mass and air constantly flows between the warm and cool areas. As such the air is better able to disperse odors. In an unstable atmosphere, the parcel of air will rise to the atmosphere’s mixing height. The volume of the atmosphere below the mixing height is called the mixing layer, and the larger the mixing layer is, the better and faster odors will disperse.
The condition when temperature actually increases with elevation is referred to as a temperature inversion. The warmer inversion layer then acts as a cap and stops vertical convective turbulence beneath it. This is considered a stable air mass and odor dispersion is constrained due to the lack of mixing. Inversion layers normally occur between 600 and 1,200 ft. in elevation, which limits the volume of the mixing layer. Temperature inversions are a result of other weather conditions in an area. They occur most often when a warm, less dense air mass moves over a dense, cold air mass. This can happen for example, when the air near the ground rapidly loses its heat on a clear night. In this situation, the ground becomes cooled quickly while the air above it retains the heat the ground was holding during the day. Additionally, temperature inversions occur in some coastal areas because upwelling of cold water can decrease surface air temperature and the cooled air mass stays beneath warmer ones.
The degree of stability of the atmosphere must be known to estimate the ability of atmosphere to disperse odors. Different methods are used for stability determination with varying degrees of complexity. Most of these methods are based on the relative importance of convective and mechanical turbulence in the atmosphere. The difference between these methods is due to use of different indicators for both convective and mechanical turbulence. When convective turbulence predominates, winds are weak and atmosphere is in unstable condition. When convection decreases and mechanical turbulence increases, the atmosphere tends to neutral conditions. Finally in absence of convective or mechanical turbulence and there is no vertical mixing, the atmosphere is in stable condition (for example, a cool morning before sunrise).
Richardson number, Monin-Obukhov length, Pasquill-Gifford stability classification and Turner stability classification are some of common methods for measuring stability. Atmospheric stability and instability are often categorized using the Pasquill atmospheric stability classes. This classification divides the atmosphere into six stability classes, with Class A being the most unstable, or turbulent, and Class F being the most stable, or least turbulent. The meteorological conditions that describe each class are shown in Table 1.
Recent developments in air emissions modeling now use the Monin-Obukhov length, rather than Pasquill stability classes, which is defined as that height at which convective turbulence is more prevalent than mechanical turbulence. EPA’s AEROMOD steady-state plume dispersion model is based on this approach. The next article in this series will examine various dispersion models in greater detail and their applicability to composting facility odor management.
Topographic Effects
Topography can affect the dispersion of odors on both a micro and mesoscale. The mesoscale effects are most pronounced in coastal areas, where differential solar heating of land and water creates the on-shore sea breezes felt during the day and the off-shore land breezes at night. Mountains also have an effect on odor dispersion, as they create greater friction to air flow from increased surface roughness, which reduces wind speeds, and they also serve as physical barriers to air flow.
Air movement in valleys takes two forms: slope winds that move downgradient into the valley and valley winds that take form along drainage ways. Cool, dense air will accumulate in the lower, flatter portions of valleys, and can intensify any thermal inversions created by radiative cooling, trapping odors in the valley. With these valley inversions, the maximum inversion depth is just before sunrise and the author has observed significant early morning valley odors in the vicinity of a biosolids composting facility in the Blue Ridge Mountains in Maryland.
Buildings in the path of a dispersing air pollution plume also impart an effect. When the plume flows over the building, a cavity of turbulent eddies is formed in the downwind side of the building. These cavities can cause increased vertical dispersion of pollutants emitted from nearby sources,resulting in elevated pollutant concentrations. If the source is located closer than five times the height of the building, the plume will be forced down to the ground much sooner than it would if no impediment was present. For example, if a 25-ft. tall equipment maintenance building was located less than 125 ft. in the downwind direction from an odor source (say a forced-draft aerated static compost pile), the eddies formed behind the building could result in higher odor concentrations immediately behind that building. This suggests that buildings downwind from the composting pad should not be located close to property lines where off-site odor impacts could occur.
Depositional Effects
Odors can be dispersed through dry and wet depositional effects. Dry deposition is the removal of odors from the air plume by contact with the ground or vegetation. Wet deposition is the removal of air pollutants by rain (essentially water scrubbing). Both effects are of primary importance to composting facilities.

Figure 2. Vegetative canopies utilized for odor dispersion (recently planted and mature) (Photos by Robert Rynk)
• Dilution and dispersion of gas concentrations of odor by a mixing effect created by windbreaks.
• Deposition of odorous dusts and other aerosols to the windward and leeward sides of windbreaks (similar to how snow lies against snow fencing).
• Collection and storage (sinks) within tree wood of the chemical constituents of odors.
• Physical interception of dust and aerosols odor particles on leaves, needles and branches.
• Containment of odor by placing windbreaks fore and/or aft of the odor source.
• Aesthetic appearance.
The water scrubbing effect of wet deposition is often observed by the fresh clean smell of the air after a rain shower. Wet deposition can be enhanced through use of area water misters that are often used at composting sites to suppress dust.
Understanding the mesoscale and microclimate aspects of odor dispersion is important in composting facility siting studies, when using odor models to predict off-site intensity and direction of odor episodes, and when taking proactive means to be a good neighbor to nearby residents and businesses.
Craig Coker is a Contributing Editor to BioCycle and a Principal in the firm Coker Composting & Consulting (www.cokercompost.com), near Roanoke VA. He can be reached at cscoker@verizon.net.