Organic forms of phosphorus, such as biosolids and compost products, contain low to very low levels of water extractable phosphorus, but increasingly are regulated like inorganic P sources.
Ron Alexander
BioCycle December 2016

Excess amounts of phosphate can cause algae blooms to occur. Inorganic forms of phosphate pose the greatest risk to water quality. ©iStockphoto.com/Sloot
Phosphorus (P) or phosphate fertilizers are sold in the marketplace by their amount of pentavalent P in the material, which is calculated as phosphorus pentoxide (P2O5). On a fertilizer label, P2O5 is listed as “available phosphate.” The sum of the water-soluble and citrate-soluble phosphate is considered to be the amount of phosphate available to the plant, and is the amount guaranteed on the fertilizer label. However, P is actually absorbed by plants in the orthophosphoric acid (orthophosphate) form, generally as H2PO4- or H2PO42-. The amounts of these ions in the soil solution are determined by soil pH (Thomason, 2002). Note that products such as biosolids are often tested (because of regulatory requirements) for their content of total phosphate, which can be converted to P2O5 by multiplying the total P by 2.29.
In most cases, keeping soils at a neutral (7.0) or slightly acidic pH will keep P in more water soluble forms. However, inorganic P can become unavailable when it reacts with oxides of iron, aluminum, manganese (in acid soils), or calcium (in alkaline soils) to form phosphate minerals (Cornell Co-op Extension, 2005). This makes the P less available for plant uptake and leaching.
Rock phosphate is the raw material used in the manufacture of most commercial phosphate fertilizers on the market. In the past, ground rock phosphate itself had been used as a source of P for acid soils. However, due to low availability of phosphate in this native material, high transportation costs, and small crop responses, very little rock phosphate is currently used in agriculture (Rehm, et al, 1997). Today, most commercial phosphate fertilizers (inorganic) begin with production of phosphoric acid from the phosphate rock, and culminate with production of orthophosphoric acid (orthophosphate), which is concentrated into liquid or dry forms of fertilizer. Inorganic forms of phosphate are essentially derived from orthophosphate, which is highly mobile as it contains a large percentage of P in “water extractable” form, also know as water extractable phosphorus (WEP). Since WEP testing measures the portion of P that is water-soluble, inorganic forms of phosphate pose the greatest risk to water quality.
Organic phosphate fertilizers have been used for centuries as the phosphate source for crops. Even with the advent of phosphate fertilizer technology processes, organic phosphate sources from animal manures, composts and biosolids (sewage sludge) are still very important (Rehm, et al, 1997). The phosphate contained in organic phosphate sources is actually a combination of both inorganic and organic phosphate.
The Problem
The excessive application of animal manure over time, and in some cases, the excess application of P-based fertilizers, has led to P migration into waterways and even ground water. Excess amounts of phosphate in fresh water streams and lakes can cause algae blooms to occur; algal blooms comprised of blue-green alga (cyanobacteria) can produce toxins that contaminate drinking water. Further, when algae die, their decomposition results in oxygen depletion, which can lead to the death of aquatic plants and animals. This process is called eutrophication (Cornell Co-op Extension, 2005), an incredibly serious problem that must be managed through greater and more responsible nutrient management and erosion control.
To better protect ground water, the application of both animal manures and biosolids will likely be governed by the P requirements of the crop to be grown, and not just the nitrogen (N) requirements. Although both manure and biosolids typically contain more N than P, the overall ratio is shallow, which means that P is applied in excess (luxury) amounts when these materials are applied to meet the N demand of the crop. Excess P application does not typically harm plants, but it can be an environmental concern.
Based on U.S. EPA data, there are 50,000 impacted waterways/water bodies in the U.S. that surpass their allowable Total Maximum Daily Loads (TMDL) for pollutants (e.g., nutrients, sediment, etc.). Further, there are many “hot spots” around the county where overproduction of animals, often in confined animal feeding operations (CAFO), has led to over application of manure to such a degree that N and P leach out of the soil and into groundwater. The causes of these hot spots, however, are much different than the cause of most of the impacted surface waters. These surface waters are primarily impacted by sediment loading, whereby erosion carries soil particles (sediment) and the nutrients (and other contaminants attached to it) to surface waters.
Thus, there are two major problems — contamination by erosion and by overfertilization — which must be managed in very different ways. Most groundwater contamination problems must be managed by better limiting the over application of manure, while most surface water contamination problems must be managed by applying appropriate erosion control measures.
Phosphorus Regulation
With significant environmental problems already existing, and future issues looming, many states have created regulations restricting the application of phosphate fertilizer to some degree. The concept of enacting regulation to reduce over or improper fertilization is a good thing, especially creating proper setbacks from surface water, avoiding application of fertilizer on frozen or saturated land, or getting it on paved or impervious surfaces.
However, implementation of the enacted regulations (and the products targeted within them) is often greatly problematic. These new regulations not only significantly impact chemical or “inorganic” phosphate usage, but in many cases, also the use of recycled organics-based products (e.g., biosolids and manure based dried fertilizers, and various composts). It should be noted that several states have exempted recycled organics-based products from nutrient management regulations, but many have not.
Based on data compiled by the Association of American Plant Food Control Officials (AAPFCO), 16 states (Connecticut, Delaware, Florida, Illinois, Maine, Maryland, Massachusetts, Michigan, Minnesota, New Hampshire, New Jersey, New York, Vermont, Virginia, Washington and Wisconsin) have enacted P fertilizer restrictions for turf. Generally, these regulations primarily impact turf managed by landscapers and homeowners. Interestingly, in most states, these regulations exempt agricultural land in production, golf courses, sod production and gardening. Many regulations allow use of phosphate fertilization on P deficient turf (if appropriately soil tested) and allow usage in turf establishment.
In response to state concerns, AAPFCO has developed recommended language regarding “Fertilizer Restrictions for Urban Landscapes,” as well as other related SUIPs (Statements of Uniform Interpretation and Policy) to assist states in developing science-based regulation. Unfortunately, many states have been overzealous in their regulation, e.g., almost eliminating even maintenance applications of P on turf, while some have not dealt with the “real” primary causes of their nutrient contamination (e.g., over fertilization or excess manure application on agricultural land, lax enforcement of NPDES Phase II regulation on sediment control during construction). Other states have unfortunately ignored the science, regulating all phosphate sources the same (i.e., ignoring their actual mobility).

For biosolids products, water extractable phosphorus is dependent upon how the biosolids are treated (biosolids pellets shown).
Data On Compost And Phosphorus Movement
A great deal of university research data exists illustrating the fact that the majority of biosolids-based products, regardless of their form (e.g., dewatered, composted, dried/granulated), contain low to very low levels of water extractable phosphorus (WEP). Therefore, these products pose lower risk to the environment. It should be noted that biosolids treated through a biological phosphorus removal (BPR) or similar processes, possess higher levels of WEP, since they concentrate P into the solid fraction of biosolids and in a more water soluble form. This same research also illustrates that:
• P movement and WEP levels are much greater in chemical or inorganic forms of P fertilizer.
• WEP levels in manure products are greater than biosolids, but much less than inorganic P fertilizer.
• Testing products for total P content is much less reliable than testing for WEP content when attempting to predict P movement (leaching).
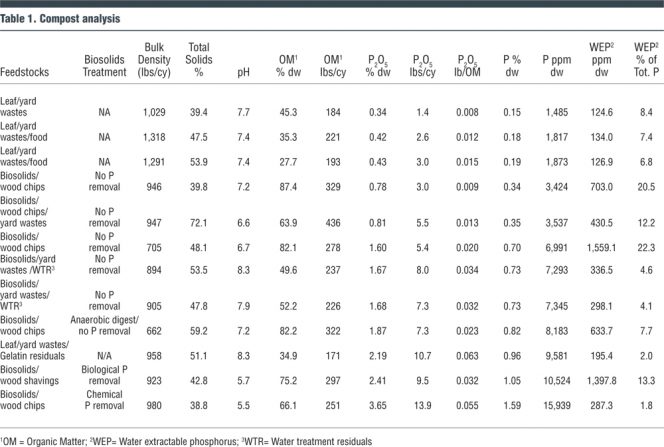
• Noticing that less data existed on the WEP content of compost products, and facing new P regulations in Massachusetts, Agresource, Inc. collected a series of compost samples and had them tested through The Penn State University testing laboratory. The test results are summarized in Table 1. Samples are presented in order from lowest to highest levels of P content on a dry weight basis. The following are interpretation of the analytical data provided by Dr. Geoff Kuter, Agresource, and Dr. John Spargo, The Pennsylvania State University.
As shown in Table 1, the amount of P, whether P2O5 or WEP, in the compost varies significantly among the 12 samples — about a 10-fold difference between the lowest and highest values. In contrast, for total nitrogen, the difference from lowest to highest is only about 4-fold, and for potassium the difference is only about 6-fold. The amount of P that is water extractable is low in comparison to the total P. The WEP ranges from about 2 percent to 22 percent of the total P.
The amount of WEP is only loosely correlated with total P. As expected, the leaf composts have lower total P and generally lower WEP than the biosolids composts. This difference between the leaf composts (the first three samples) and the biosolids composts is entirely expected. However, among the various biosolids composts there is a wide range of both total P and WEP, and some of the biosolids composts with the highest levels of total P have among the lowest levels of WEP.
The percentage of P that is water extractable is very closely correlated with the amount of iron (Fe) and aluminum (Al) in the individual product. The data set supports other studies that indicate that for biosolids products, WEP is dependent upon how the biosolids are treated. The wide range in total P and WEP among the different biosolids composts is likely due, at least in part, to the variations in P removal practices performed at the different treatment plants. For example, the plant with biological P removal has a relatively high WEP in comparison to the plant that performed chemical P removal through the addition of Al and Fe. The biosolids compost that includes small amounts of water treatment residuals had relatively high levels of Al, and thus, had among the lowest levels of WEP for the biosolids composts.
The data set includes a sample made with gelatin manufacturing residuals and is somewhat anomalous and adds an interesting twist to the data. The gelatin residuals are largely a biological sludge high in both calcium (Ca) and P. This compost is similar to the biosolids composts; it has a relatively high amount of total P, but has among the lowest levels of WEP. In this particular compost, the very high levels of Ca, in addition to Al and Fe, are likely the cause of the low percentage of WEP. There are a number of other factors, aside from just total P, Fe, and Al that are controlling WEP — and Ca is probably an important variable. The other factor is the mineralogy of the Fe and Al. The more poorly crystalline Fe and Al oxides (sesquioxides) are far more reactive. Unfortunately, Penn State was not able to set up and run acid ammonium oxalate extractable Fe and Al, which would give a measure of the reactive P sorbing mineral surfaces.
Soil Type Impact
If we assume that WEP is a predictor of P losses, the data indicates that some biosolids composts, despite the relatively high total P, could be used to build soil organic matter levels with no more risk than the leaf composts that have lower total P. For example, in some of the biosolids composts, the WEP per pound of organic matter is no greater than among the leaf composts. While there is little doubt that WEP is a good predictor of runoff P loss from surface applied biosolids, it may not be so simple for incorporated compost. Once the compost equilibrates with the soil, the reactivity of P, Fe, Al, and Ca are going to change, driven by soil mineralogy, soil moisture, acidity, enhanced microbial activity, etc. There is no question that Fe, Al, and Ca content are going to have an impact, but it is also going to be significantly influenced by soil properties. Further, runoff P loss is a function of both source (e.g., P concentration and solubility) and transport factors (e.g., soil structure, water permeability, slope, slope length). Therefore, the amount of organic matter applied with compost is also going to play a big role in mitigating risk of P runoff losses.
Although the data shows that some composts have high Al and Fe (and Ca) levels, so as to reduce the WEP levels, it is not clear that these levels reduce WEP levels to the point that P is unavailable to plants or that soluble P contributed from storm water would be bound to the degree that would eliminate all potential for leaching. For this reason, testing of sand-based mixes containing compost and used for various storm water applications should be tested for these nutrient indicators of P movement, as well as nutrients in the leached water.
These data illustrate that not all composts are the same, with regards to their composition. Further, it is apparent that measuring and considering only total P in a compost (as well as other recycled organics products) is not likely to be the best indicator of P mobility or potential leaching into vulnerable water sources.
Final Thoughts
Both P and N removal and concentration processes should be more widely used in biosolids and manure management. These technologies will allow recycled organic products to be used more effectively as soil amendments (for their carbon/organic matter content), as well as allow their nutrient components to be used more precisely.
Future research and regulation must focus on P application as it relates to WEP, and not just total P. Regulations must also consider the research that has shown how the addition of organic matter, and improving “soil health” (improved structure and biology), impact the movement and utilization of P (and other nutrients). It appears that research data also supports a “rethink” of current P restriction regulations. Certainly, regulators must be urged to consider the WEP content of the products and use appropriate Phosphorus Source Coefficients (a measure of the relative availability of P to be lost in runoff) in their regulation (and BMPs), as well as consider how and if P indexing should be used as a regulatory tool. Further, regulations that virtually eliminate P usage in turf maintenance must be reconsidered. There is concern that turf which is not provided maintenance amounts of P will cause it dieback (become thin). Poor turf quality would create more exposed soil which is likely to erode, further increasing nutrient migration and soil losses.
State lawmakers must further be educated about the science pertaining to P utilization and movement, and how it — and soil erosion — can impact water quality. Further, legislation must avoid impacting use of recycled organic products, primarily those with a low WEP content, as the these products provide important benefits to mitigating the impacts of climate change, including reduction of water usage, improved erosion control and less expensive storm water management. The ability of these “green” products to enhance overall soil health, which can also improve plant establishment and improve food production, must not be overlooked.
Ron Alexander is president of R. Alexander Associates, Inc. (Apex, North Carolina, 919-367-8350, www.alexassoc.net), a company specializing in product and market development for organic recycled products. He is author of ‘The Practical Guide to Compost Marketing and Sales’ (2nd Edition published by BioCycle) and has over 30 years of experience in compost and organic recycled product marketing. Mr. Alexander serves as co-chair of the US Composting Council’s Market Development Committee and is an Industry Liaison to AAPFCO (Association of American Plant Food Control Officials). This article was edited by Dr. Geoff Kuter, Agresource, Inc.