Craig Coker
Fires at composting and mulching facilities happen frequently. A minor fire threatens to attract public inquiry about the risks and nuisances of mulching or composting activities at a particular site, and within the industry generally. A major fire threatens ongoing operations and presents a potential danger to workers and firefighters. Fires can be caused by human error (e.g., a carelessly-discarded cigarette), improper procedures (e.g., a welding spark from doing equipment repair next to a pile of material), lightning, arson, wildfires and spontaneous combustion. This article examines this last cause.
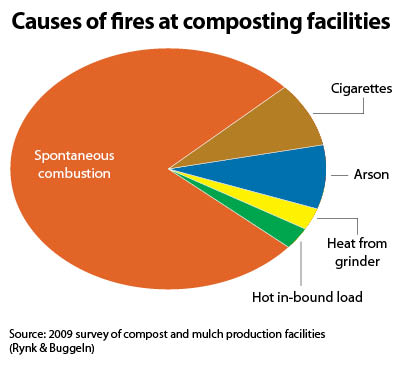 Spontaneous combustion (SC) has been observed and documented in numerous materials derived from living plants and animals, including agricultural materials (hay, straw, cotton, grains), animal fibers and hides, energy materials (coal), food products, fertilizer products (alfalfa meal, bone meal, fish meal), woody materials of all sorts, and, of course, compost and mulch piles. SC is defined as the combustion of material in the absence of “forced ignition,” or an external source of spark or flame (Buggeln & Rynk, 2002). SC occurs because temperatures rise past a critical setpoint due to the rate of heat loss in a pile being less than the rate of heat build-up.
Spontaneous combustion (SC) has been observed and documented in numerous materials derived from living plants and animals, including agricultural materials (hay, straw, cotton, grains), animal fibers and hides, energy materials (coal), food products, fertilizer products (alfalfa meal, bone meal, fish meal), woody materials of all sorts, and, of course, compost and mulch piles. SC is defined as the combustion of material in the absence of “forced ignition,” or an external source of spark or flame (Buggeln & Rynk, 2002). SC occurs because temperatures rise past a critical setpoint due to the rate of heat loss in a pile being less than the rate of heat build-up.
Heat Sources
Almost all composting piles are built from a mixture of nitrogenous and carbonaceous materials. Most composters use vegetation as a carbon source and bulking agent to provide free air space. Cutting down vegetation doesn’t cause instant death to the plant cells that make up the vegetation. They continue to respire until their food and/or moisture are exhausted or until they encounter lethal conditions like high temperatures. These heat sources from respiration of plant cells and microflora are considered biotic heat. This heat is produced by the citric acid cycle, which is a series of enzymatic reactions that convert glucose to carbon dioxide, water vapor, chemical energy (in the form of ATP, or adenosine triphosphate), and heat. If you’ve ever noticed how quickly a pile of grass clippings will heat up, that’s due to the respiration of those still-living plant cells in the grass clippings. With adequate moisture, plant cell respiration contributes heat until temperatures rise to lethal levels, which are between 25°C and 50°C (77°F-122°F) (Buggeln & Rynk, 2002).
When plant cells die, they release various water-soluble cellular metabolites, such as sugars, amino acids, and organic acids. These, in turn, are food for the bacteria that cover vegetative surfaces. For woody, or lignocellulosic materials, the metabolites are food for the fungi that degrade lignin. This population of microflora in turn respires, converting glucose and oxygen into carbon dioxide, water vapor and heat.
Another source of heat in composting piles is the direct oxidation of plant chemicals that react with the oxygen in the pile. The chemicals most subject to direct oxidation are the lipids commonly found in plant cells, including palmitic, oleic, linoleic and linolenic acids. Linolenic acid is the basis for linseed oil, a wood finishing treatment. Woodworkers know not to put linseed oil-soaked rags in an enclosed bucket as SC will occur at room temperatures! Other plant resins and oils are not as reactive but it is thought that these resins can oxidize at lower temperatures while inhibiting biological degradation. This may be one reason why coniferous tree wood chips decompose more slowly than hardwood chips. This source of heat is considered abiotic heat. Heat generation due to abiotic “auto-oxidation” is detectable at about 80°C (176°F) but can occur at lower temperatures in the presence of moisture, similar to how moisture accelerates the oxidation or rusting of iron (Buggeln & Rynk, 2002).
There is a third source of heat in composting piles. As temperatures rise, heat can be generated by pyrolysis, defined as the chemical decomposition of a material by the action of heat in an oxygen-starved environment. There are places in composting piles that can be oxygen-starved, as not every cubic foot of a composting pile is well-oxygenated all the time even with aerated static pile (ASP) composting systems. Pyrolysis is a heat-derived chemical process, where cellulose and lignin are broken down from long to short carbon structures. Pyrolysis gas and char are products in the pyrolysis process.
Some of these gases produced can react with oxygen to produce heat. The decomposition of organic molecules at temperatures of 65°C to 70°C (149°F-158°F), maintained for weeks or months, in the presence of water and oxygen, results in the production of water, carbon dioxide and heat. As the carbon dioxide dissolves in the pore water of the composting pile, the pH of the water decreases. This formed weak acid further accelerates the decomposition of organics, producing additional acids (Buggeln & Rynk, 2002). This process is known as slow pyrolysis, in contrast to the more well-studied high temperature pyrolysis associated with chemical waste conversion technologies. The heat transfer rate in slow pyrolysis is 5°C to 7°C/minute; in fast pyrolysis, the heat transfer rate is more than 300°C/minute (Gustafsson, 2013).
Chain Reaction
In short, composting piles are subject to heat buildup from biotic, abiotic and pyrolytic sources. This heat buildup is eased by heat losses due to evaporation of moisture, by either passive or forced aeration, and by mechanical turning. If the moisture content of a pile becomes too low, the effectiveness of the pile to cool due to evaporation of moisture declines, which allows temperatures to climb to 60°C (140°F) and above (Alberta, 2006). But SC is a chain reaction of a combination of biotic, abiotic and pyrolytic factors. Each process in the chain raises the temperature to the level where the next process takes over and raises temperatures higher until the pile ignites. Each succeeding process advances faster than the previous one and each process speeds up as temperatures rise. The rule of thumb states that the reaction rate roughly doubles with each 10°C (18°F) rise in temperature. So heat is released about 16 times faster at 100°C (212°F) than at 60°C (140°F). As SC progresses through the steps in the chain, there is less and less time to react and halt it (Rynk, 2000a).
The transition from biotic to abiotic heat-producing processes occurs at about 80°C (175°F) but, as noted previously, can be a bit lower in piles with some moisture but not enough moisture to cool the pile. The initial abiotic process is the direct chemical oxidation of dry materials. The speed of chemical oxidation increases as temperatures increase and it requires a continued supply of oxygen to continue, and will continue even under low-oxygen concentrations. Chemical oxidation will stop in the absence of oxygen, which is why smothering a SC smoldering fire with dirt can potentially put out the fire (although this may not stop a SC fire, which is discussed in Part II). In large piles of organic materials, with limited available oxygen, SC usually leads to a smoldering fire, generally at temperatures between 150°C to 200°C (300°F-400°F). If enough oxygen is suddenly supplied by the aeration system or by opening up the pile, temperatures will increase immediately and dramatically to create a flaming fire (Rynk, 2000a).
Part II of this series cover how to catch the beginnings of a spontaneous combustion fire and fight it successfully.
Craig Coker is a Senior Editor at BioCycle CONNECT and a Principal in the firm Coker Composting & Consulting (www.cokercompost.com), near Roanoke VA. He can be reached at ccoker@cokercompost.com.













